Khaberni - AstraZeneca announced on Tuesday that the US Food and Drug Administration has rejected its application for approval of an easier-to-use version of the lupus treatment "Saphnelo". As a result of this decision, any potential approval will be postponed until the first half of 2026.
The Anglo-Swedish company explained that the agency issued what is known as a "complete response letter" regarding the new version that is administered through subcutaneous injections, which negatively impacted the performance of the company's stock, dropping by more than 1.5% in early trading.
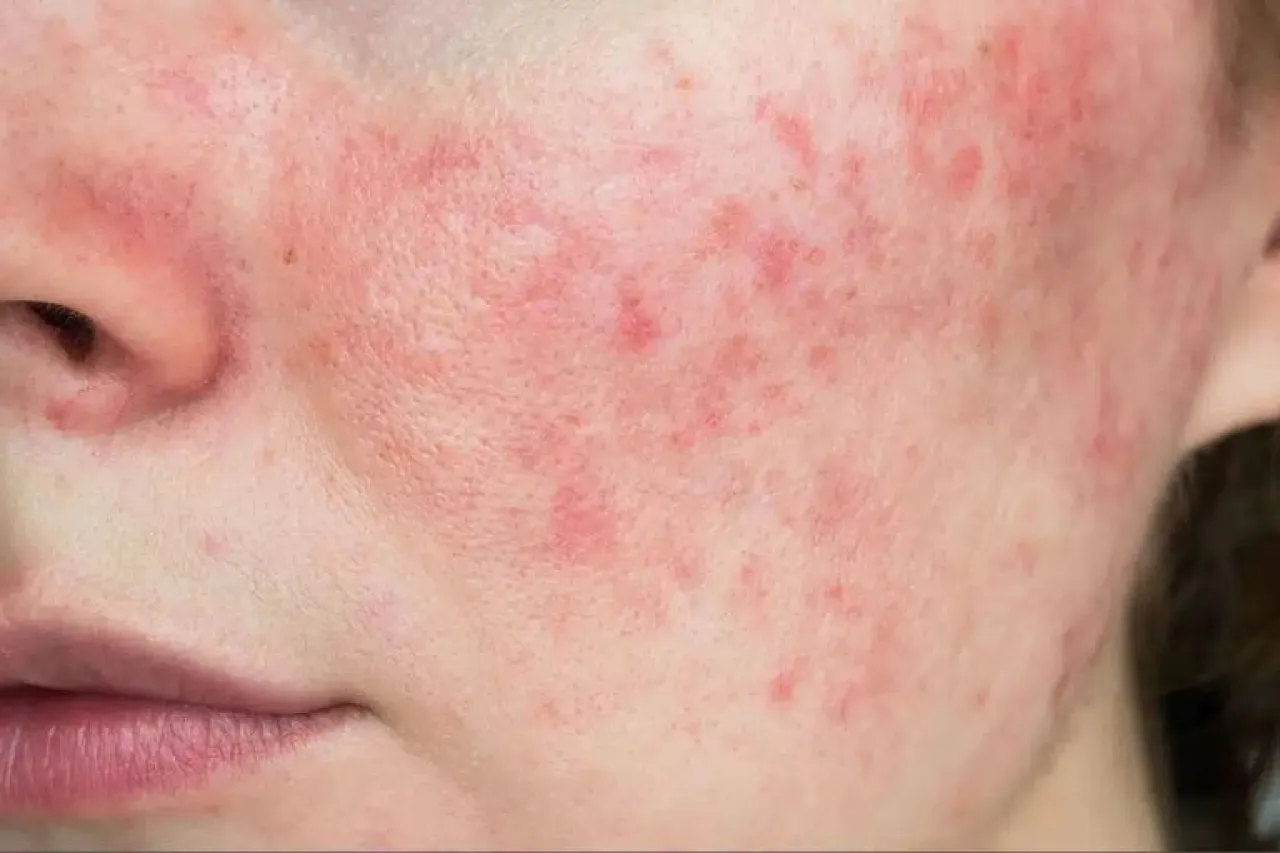
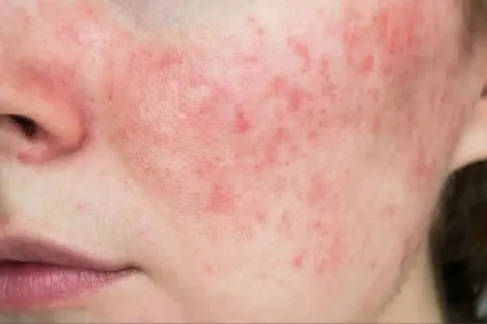
This US decision contrasts with the position of the European Union, which recently approved the use of the subcutaneous form of the Saphnelo drug for treating adult patients with moderate to severe systemic lupus erythematosus, a chronic autoimmune disease that affects more than 3.4 million people worldwide.
The new version of the treatment is characterized by the possibility of self-injection at home, instead of being administered through intravenous solutions in hospitals or clinics, providing patients with greater flexibility and ease of treatment.
AstraZeneca confirmed that it has already provided the additional information requested by the US agency and will continue to work with the "US Food and Drug Administration" to advance the approval file, while the intravenous form of Saphnelo, currently approved in more than 70 countries, remains available in the market.
The US agency's rejection came despite the treatment achieving its primary goal during an advanced clinical trial, where it demonstrated a remarkable ability to reduce the activity of systemic lupus erythematosus, which occurs when the immune system attacks healthy body tissues and may lead to organ failure in some cases.
Meanwhile, AstraZeneca continues to expand the use of Saphnelo through clinical trials for other chronic autoimmune diseases, including cutaneous lupus, myositis, systemic sclerosis, and lupus nephritis.
Under an updated licensing agreement in 2025, the company will pay royalties to Bristol Myers Squibb for drug sales in the United States.
Saphnelo treatment generated revenues of $483 million during the first nine months of 2025, representing about 1% of AstraZeneca's total revenues during that period.