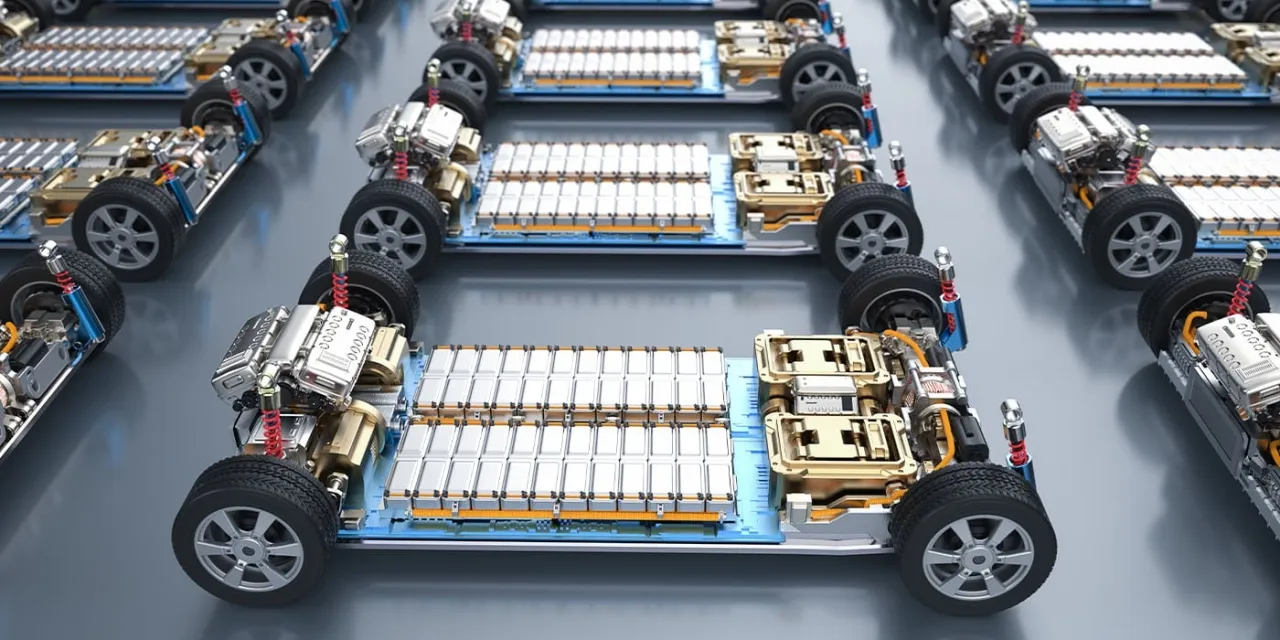
Khaberni - A team of scientists in South Korea has developed an environmentally friendly recycling process for electric car batteries that has managed to extract more than 95% of nickel and cobalt elements from old batteries with nearly complete purity reaching 99%, representing a scientific achievement that may reshape the industry of lithium-ion batteries globally.
The results of the study were published in the journal Energy Storage Materials, supported by the South Korean Ministry of Education, the National Research Foundation of Korea, and UNIST University.
This innovation was made by researchers from the Ulsan National Institute of Science and Technology (UNIST), who developed a selective chemical separation technology based on electrolysis using a special multifunctional solvent, without the need for complex chemical treatments or producing large amounts of hazardous wastewater.
The aviation sector in 2025 witnessed a noticeable shift towards the adoption of electric aircraft, with promises of quieter, more efficient, and cleaner flights.
Professor Kyung Kim, from the Department of Civil and Environmental Engineering at the institute, explained that the new technology overcomes the old dilemma between metal purity and recovery rate in electrochemical separation processes, making it a sustainable and cost-effective solution.
The consumed lithium-ion batteries are known as "Urban Mines" due to their content of vital metals such as nickel (Ni), cobalt (Co), and manganese (Mn), although the presence of these metals together poses a significant challenge in the effective separation process.
Traditional methods typically rely on strong acids such as sulfuric acid, alongside multiple step chemical extraction processes, making them costly and environmentally polluting.
To overcome this, UNIST scientists used a solvent known as "ethaline," a mixture of ethylene glycol and chloride ions, which selectively binds to nickel ions, while chloride ions form stable compounds with cobalt known as tetrachlorocobaltates.
This process allowed for high precision separation of the metals, with nickel precipitating at –0.45 volts and cobalt at –0.9 volts, creating a precise electrochemical window that enabled separate and remarkably efficient metal recovery.
Are you looking for a luxury sports car for children? In this case, just look for this miniature version of the Mercedes-Benz SL300 priced at ($49,000).
The results showed that the team maintained a constant voltage difference between the metals even at high temperatures reaching 85 degrees Celsius, achieving a separation factor of over 3000 and recovering more than 97% of the nickel from synthetic samples.
When the technique was applied to actual nickel-cobalt-manganese (NCM) battery solutions, the purity reached 99.1% for nickel and 98.8% for cobalt, with recovery rates exceeding 95%.
The electrolysis process also contributed to the automatic purification of nickel due to the formation of chlorine in the solvent, without the need for additional stages, while the solution can be safely reused after neutralization, reducing environmental impact and operational costs.
Professor Kim confirmed that the new method reduces the use of chemicals and minimizes liquid waste, contributing to a more sustainable battery recycling ecosystem.