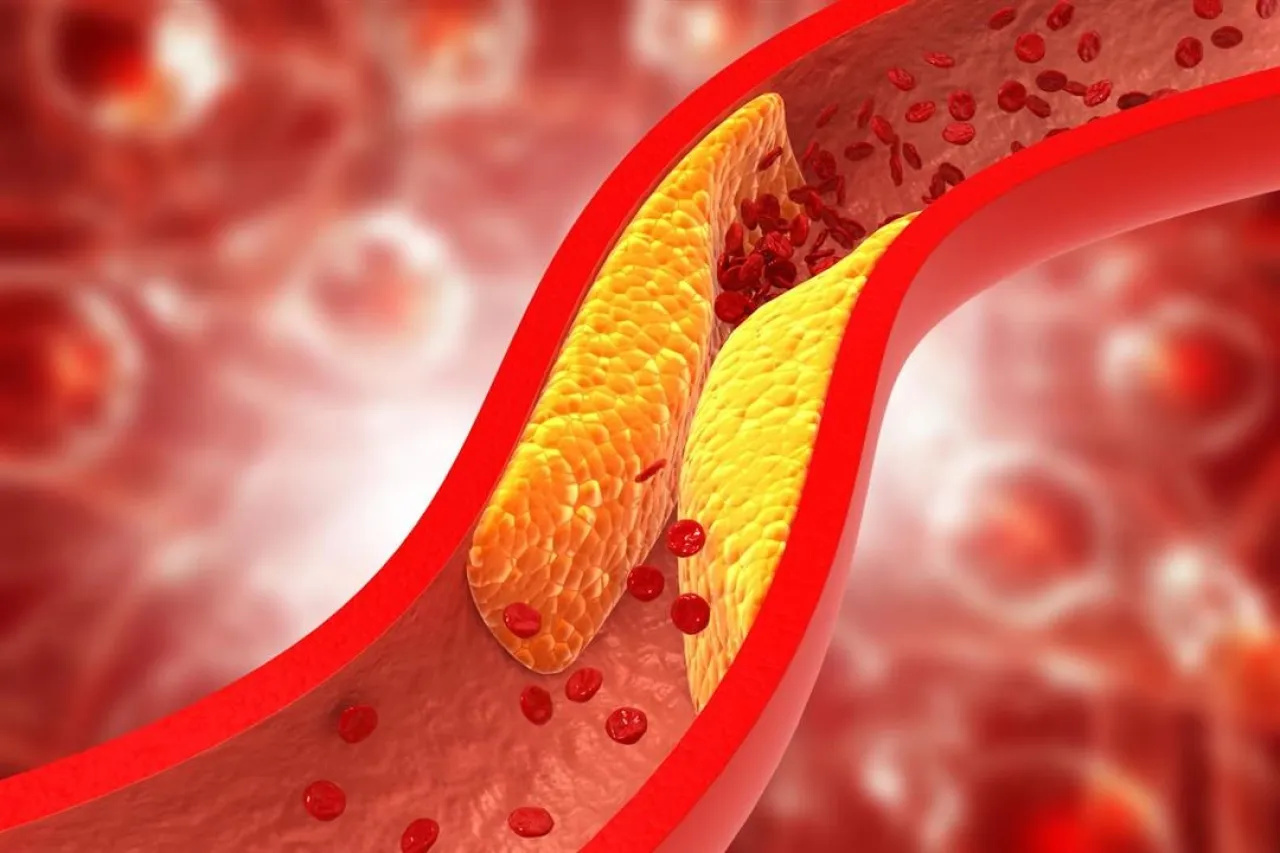
Khaberni - A single injection of an experimental gene therapy from "CRISPR Therapeutics" has been shown to be safe and reduce levels of harmful cholesterol and triglycerides by half in four individuals who received the highest dose, raising hopes for single-dose therapy.
Cardiologist Steven Nissen from the Cleveland Clinic, the lead researcher in the first study of the treatment in humans, said, "We had nothing that reduced both harmful cholesterol and triglycerides by nearly 50 percent."
Dr. Luke Laffin from the Cleveland Clinic, a co-researcher in the study, mentioned that although the development of this therapy is still in very early stages, future trials demonstrating that treatment "CTX310" is safe and effective could change medical practices.
"Instead of taking a pill once a day or getting monthly injections, this treatment could provide a safe and permanent once-only injection for patients suffering from high cholesterol," he said.
The findings were presented on Saturday at the American Heart Association meeting in New Orleans and were published in the New England Journal of Medicine.
High levels of low-density lipoprotein, or "harmful" cholesterol, may lead to the accumulation of deposits in the artery walls, increasing the risk of heart attacks or strokes. High triglycerides, another type of fat in the blood, may also increase these risks.
Shutting Off the Gene
"CTX310" works by shutting off a gene called "ANGPTL3" through a single two-hour injection. It is inspired by studies showing that those born with an inactive version of the "ANGPTL3" gene have lower lifetime heart disease risks without any noticeable negative consequences.
The drug "Evinacumab" from Regeneron, which treats a rare genetic disorder, targets the same gene but requires monthly injections.
The CRISPR trial conducted in Australia, New Zealand, and the United Kingdom involved 15 patients aged between 31 and 68, testing five different doses on them. All participants suffered from high triglycerides or high "harmful" cholesterol levels or both and had failed to respond to other treatments.
Among the four patients who received the highest dose, triglycerides decreased by an average of 55 percent and harmful cholesterol by 50 percent two weeks post-treatment. The levels remained low for at least two more months.
Nissen said, "We will try to prove the safety and effectiveness of these once-only treatments because we believe these options are important for patients."
Laffin mentioned that three participants had temporary reactions to the treatment, including nausea and elevated liver enzymes, which quickly disappeared.
The participants will be monitored for a year after the trial, with the option to follow up for an additional 15 years.