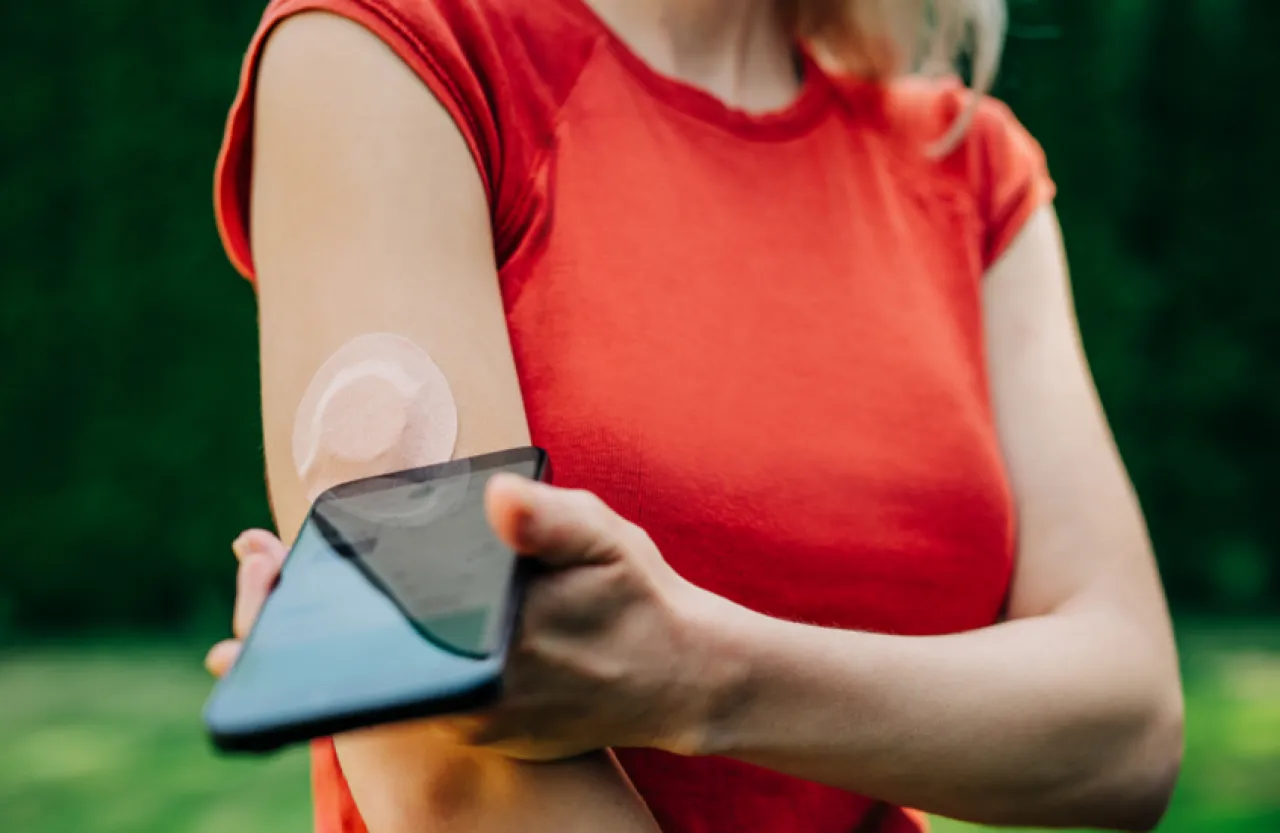
The multinational healthcare company Abbott has recalled millions of blood glucose sensor devices of the types FreeStyle Libre 3 and FreeStyle Libre 3 Plus.
This comes after the discovery of a technical malfunction that could lead to inaccurate readings linked to serious health risks, including seven deaths and 736 injuries (among them 57 injuries in the United States).
The company reports indicate that the defect stemmed from only one production line among several that manufacture these sensors. Approximately three million defective sensors have been distributed in the United States, in addition to millions more in many other countries including Austria, Belgium, Canada, Denmark, Finland, France, Germany, Italy, Luxembourg, The Netherlands, New Zealand, Norway, Spain, Sweden, Switzerland, and the United Kingdom.
A large segment of diabetes patients relies on these devices to ensure accurate glucose level monitoring, as a falsely elevated reading can lead to ignoring the treatment for hypoglycemia, exposing the patient to severe hypoglycemia. Conversely, falsely low readings may cause excessive doses of insulin to be administered, which also leads to serious drops in blood sugar levels.
Failure to detect a true increase in glucose levels may lead to diabetic ketoacidosis, a life-threatening complication.
The company explained that the sensor's operation mechanism relies on inserting a thin thread covered in a special enzyme under the skin to measure glucose in the interstitial fluid, where the glucose concentration is converted into an electrical current measured electronically and the readings are transmitted to the user's phones.
Abbott explained that samples taken from the affected production line showed inaccurately low readings, confirming that continued reliance on them for extended periods could lead to incorrect therapeutic decisions, such as overconsumption of carbohydrates or skipping insulin doses, which can cause serious injuries or even death, in addition to long-term complications including nerve damage, kidney diseases, and cardiovascular problems.
The company confirmed that it had identified and successfully addressed the cause of the defect, indicating that it continues to produce Libre 3 and Libre 3 Plus sensors to meet new and replacement orders without expecting a significant supply shortage.